Jmol Tutorial 3
- Home
- Jmol Tutorial 1
- Jmol Tutorial 2
- Jmol Tutorial 3
- Jmol Commands
- Resources Pages
- PDB Tutorial
- SMART Teams
Symposium
Part 3 - Exploring Quaternary Structure - The Insulin Hexamer
1. Identifying Zinc
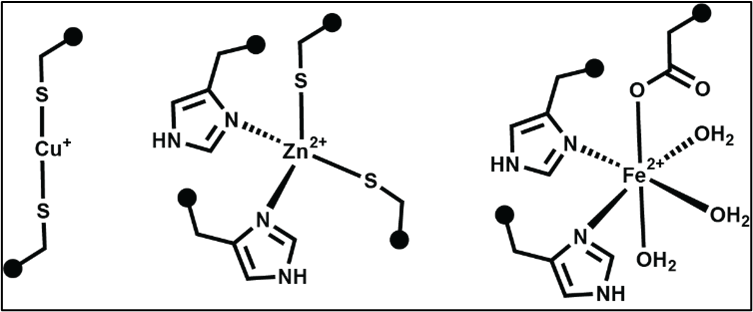
Metal ions form coordination bonds with amino acids. These interactions are similar in strength to covalent and ionic bonds. The amino acid side chain needs to have a pair of electrons – O, N, or S atom – to coordinate a metal ion.
Each type of metal ion prefers to bind certain amino acids and in certain bonding arrangements. Some examples are shown below.
How would you know that insulin uses a small molecule (Zinc) to stabilize its quaternary structure? You can check out the Protein Data Bank (www.rcsb.org) to find this information.
Go to www.rcsb.org. In the search bar on the type right of the webpage, search for the PDB file: 1tyl.
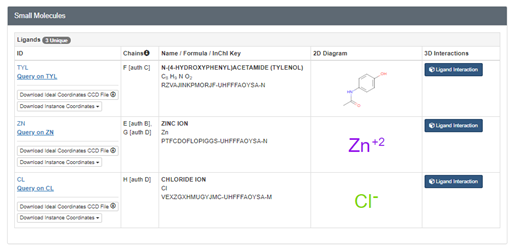
The search will take you to the Structure Summary page. Scroll down the page to find the box labeled “Small Molecules.” This shows you the other molecules that are important for the overall structure of the insulin hexamer. In this case, you can see that one of the important small molecules is zinc. Insulin forms important coordination interactions with the metal ion, Zn(II).
Next, you will identify the zinc binding sites in the PDB file 1TYL and show zinc in spacefill format.
To access JUDE, go to:
https://www.centerforbiomolecularmodeling.org/modelingResources/jmolUserDesignEnvironment/#forward.
To select the Zinc ion and color it green:
select zn
spacefill 1.25
color green
Zoom in on each zinc site and identify all coordination partners. Mouse over each bonding partner to view amino acid name, atom name, chain ID, etc.
Questions:
1. Which chains do the zinc-coordinating amino acids originate from?
2. Which atom of the side chain interacts with zinc?
3. Is there anything else bound to the metal?
4. Measure the distance between the side chain and metal ion for each zinc site. Record the distances in units of Å. How do these distances compare to typical values for covalent bonds, ionic bonds, H-bonds, etc.?
5. Double-click on the zinc ion. Mouse over to the bonding partner and click the atom coordinated to zinc. Given what we know about the requirements for zinc coordination bonds – do the interactions between zinc and insulin match our predictions for this metal?
2. Exploring Insulin Quaternary Structure
Insulin can adopt different quaternary structures. The quaternary structure of a protein is defined by the number of protein chains and how they are arranged. In this tutorial, we will view a common insulin structure used for storage of the protein and evaluate the role of zinc in this form of insulin.
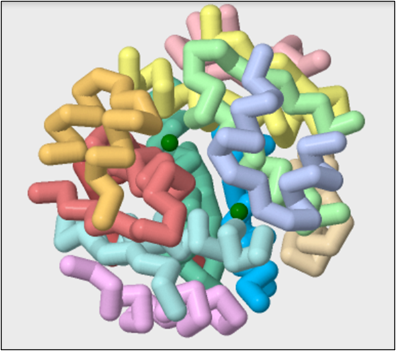
First, we will view the quaternary structure of insulin in the PDB file 1EV6. We will show the protein in backbone format using the commands below and color each chain a different color.
On JUDE, Load public PDB file 1EV6.
backbone only
backbone 1.5
color chains
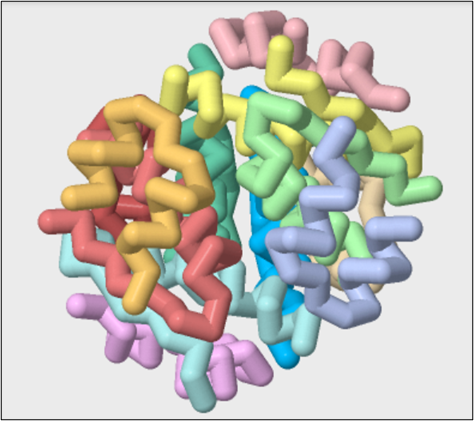
Questions:
6. Count the number of A and B chains and describe how they are arranged in space.
7. Identify the zinc sites in this form of insulin.
select zn
spacefill 1.25
color green
Questions:
8. Describe the number of bound metal ions and their location in the structure.
9. Zoom in on each zinc site and identify all coordination partners. Mouse over each bonding partner to view amino acid name, atom name, chain ID, etc.
10. Describe or draw the geometry of the Zn(II) binding site.
11. Make a prediction – does zinc stabilize or destabilize the hexameric quaternary structure of insulin? Why or why not?
12. Could another metal substitute for zinc in this function?
13. What would happen if one of the amino acids coordinated to zinc were mutated to another type of side chain?
14. Compare and contrast zinc in insulin to the function of a disulfide bond? Could a disulfide bond substitute for the metal ion in insulin? Why or why not?
